SILVER CHLORIDE
Basic information
- Chemical formula(s): \({\rm AgCl}\)
- Other names: Silver(I) chloride, chlorosilver, cerargyrite
- CAS number: 7783-90-6
- EC number: 232-033-3
- Molecular weight: 143.32 g/mol
- International Chemical Safety Card (ICSC): N/A (see ThermoFisher safety data sheet)
- Flammability: no
- Description: White crystalline solid, practically insoluble in water and dilute acids. Darkens on exposure to light due to photochemical decomposition. Used in photography, electrodes, and as a reference standard.
NFPA 704 (fire diamond)
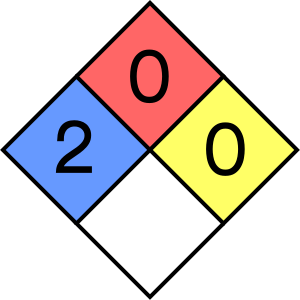
- Health (blue): 2 - intense or continued but not chronic exposure could cause temporary incapacitation or possible residual injury.
- Flammability (red): 0 - will not burn under typical fire conditions.
- Instability–reactivity (yellow): 0 - normally stable, even under fire exposure conditions, and is not reactive with water.
- Special notice (white): -
Hazard statements
| Code | Phrase |
|---|---|
| H400 | very toxic to aquatic life |
| H410 | very toxic to aquatic life with long lasting effects |
Precautionary statements
| Code | Phrase |
|---|---|
| P273 | avoid release to the environment |
| P280 | wear protective gloves/protective clothing/eye protection/face protection |
| P391 | collect spillage |
| P501 | dispose of contents/container in accordance with local/regional/national/international regulations |
Protective measures
Gloves
For handling silver chloride powder or solutions:
- Nitrile: very good - chemical resistant and prevents skin contact with silver compounds.
- Natural latex or rubber: good - adequate for routine handling.
- PVC: good - suitable for handling silver chloride.
- Butyl rubber: very good - excellent chemical resistance for extended contact.
Safety goggles
- Always wear safety glasses or goggles when handling this product.
- Use eye protection equipment that has been tested and approved by recognized national standards.
- Corrective glasses are not considered safety goggles.
- Prevent eye contact to avoid irritation.
Clothing
- Wear protective clothing to prevent skin contact.
- Long sleeves and full-length pants required.
- Closed-toe shoes mandatory.
- Remove contaminated clothing immediately and wash before reuse.
- Prevent skin contact as silver compounds may cause discoloration.
Respiratory protection
- Ensure adequate ventilation when handling.
- Use dust mask when working with fine powder to prevent inhalation.
- For large quantities or poor ventilation, use appropriate respiratory protection.
- Minimize dust generation during handling.
Spill management
- Prevent environmental release - silver chloride is very toxic to aquatic life.
- Wear appropriate personal protective equipment.
- Collect spillage using non-sparking tools (avoid metal tools that may react).
- Sweep up material carefully to avoid creating dust.
- Place collected material in appropriate container for disposal.
- Do not wash spills down drains or allow entry into waterways.
- Clean area thoroughly after material removal.
- Special disposal requirements - dispose as hazardous waste according to local regulations.
- Contact environmental authorities for guidance on large spills.
Special warnings
- Very toxic to aquatic life - prevent release to environment, waterways, or soil.
- Photosensitive - darkens when exposed to light due to decomposition to metallic silver.
- Argyria risk - prolonged exposure to silver compounds may cause irreversible blue-gray discoloration of skin and eyes.
- Insoluble in water - forms stable suspensions that can persist in aquatic environments.
- Store in dark, cool, dry place in tightly closed containers.
- Keep away from light to prevent photochemical decomposition.
- Incompatible with ammonia - forms explosive silver-ammonia compounds.
- Incompatible with cyanides - may form toxic hydrogen cyanide gas.
- Handle with non-metallic tools when possible to prevent contamination.
- Regulated waste - requires special disposal as hazardous waste due to silver content.